Featured News, News
Technology to Support and Accelerate Wound Healing
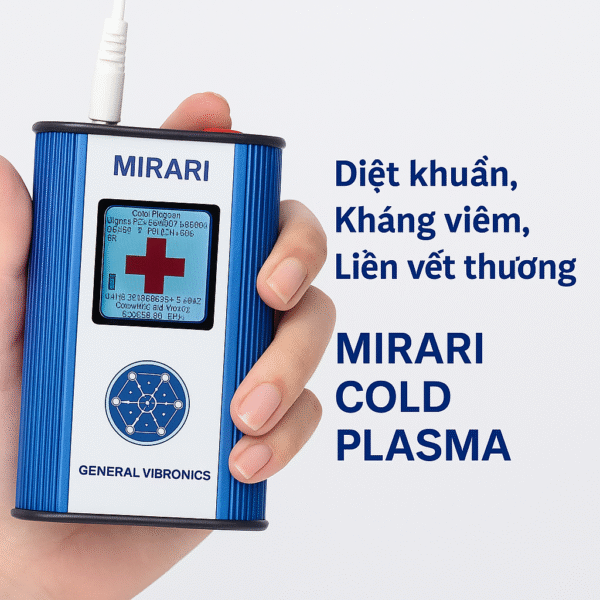
Non-invasive cold plasma technology supports accelerating the healing process, eliminating harmful microorganisms, and regenerating cells.
According to Mirari representatives, cold plasma offers a safe non-invasive solution in various healthcare fields, especially in treating hard-to-heal wounds. Additionally, the technology is applied to pain relief and musculoskeletal recovery, specifically helping reduce inflammation and pain without medication, supporting quick recovery after injury or surgery.
In skincare, cold plasma supports the treatment of acne, dermatitis, eczema, psoriasis, and healing skin lesions without causing side effects. This technology also helps control infections by killing bacteria, viruses, and fungi, protecting patients and improving treatment effectiveness in medical environments.
![Mirari Cold Plasma Device GVM2 /[Serial Number]/3.053. Photo: Mirari](https://miraridoctor.vn/wp-content/uploads/2025/02/z6313064154221-8cab706d8b62ba7-2569-2943-1739437973.jpg)
The characteristics of Mirari Cold Plasma include:
Non-thermal: The new technology does not significantly increase the temperature of the surrounding environment, suitable for application on sensitive tissues and organs.
Reactive properties: Cold plasma produces a range of reactive oxygen species (ROS) and reactive nitrogen species (RNS), helping antibacterial, antiviral, antifungal effects, promoting healing and tissue regeneration.
Antimicrobial: Cold plasma can effectively inactivate many pathogens.
Selective action: The technology can selectively target and destroy unwanted cells such as cancer cells, without harming healthy cells.
Mirari representatives state that besides using cold plasma as recommended, patients who want to speed up wound healing need to pay attention to other factors. For example, post-surgery nutrition should follow doctors’ advice, with a variety of nutrients such as vitamin A, vitamin C, protein…
To avoid infections, in addition to using new technology, everyone should care for wounds and surgical sites following medical procedures and change dressings regularly.

Mirari Import-Export Joint Stock Company is the exclusive distributor of Mirari Cold Plasma devices in Vietnam. This is an advanced solution for treating wounds, pain relief, dermatology, and musculoskeletal issues. The device, manufactured by General Vibronics USA, was approved by the U.S. Food and Drug Administration (FDA) in November 2024 (Certificate No. 3959-1-2025-1), ensuring compliance with international standards for safety and medical effectiveness.
Over the past 8 years, many suppliers have provided versions of the Mirari Cold Plasma Control Unit not approved by the FDA and not fully meeting safety standards. To verify FDA certification, users should check the information displayed when starting the control unit with the main screen showing the correct format: GVM2 /[Serial Number]/3.053.
Source: VNEXPRESS