Applications of Mirari Cold Plasma
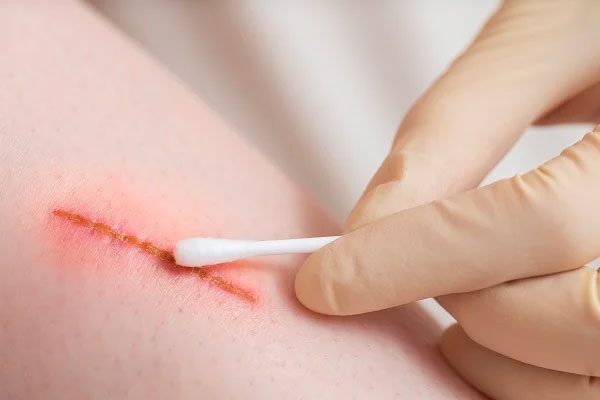
Application in Supporting Wound Healing
MIRARI COLD PLASMA APPLICATION
IN SUPPORTING WOUND HEALING
1. General Information
Mirari Cold Plasma is a cold plasma generator. Cold plasma is a low-energy state of matter containing charged particles, activated molecules, and safe-level ultraviolet radiation. When applied to skin/tissue, cold plasma can kill bacteria, reduce inflammation, promote new tissue regeneration, and relieve swelling and pain without thermal damage, making it effective for musculoskeletal disorders, wounds, dermatology, and rehabilitation.
Mirari Cold Plasma is certified as a Class B medical device in Vietnam and has received FDA certification in the United States to ensure quality and safety standards.
Mirari Cold Plasma is developed by General Vibronics in the USA and exclusively distributed in Vietnam by Mirari Import-Export Joint Stock Company.
2. Applications of Mirari Cold Plasma in Supporting Wound Healing
The Mirari Cold Plasma generator is designed to support wound healing, including:
| Type of Condition | Clinical Features | Effects of Mirari Cold Plasma | Clinical Evidence |
| Diabetic Foot Ulcer | Chronic foot ulcers due to circulatory and neurological disorders | – Kills bacteria, including antibiotic-resistant strains – Reduces inflammation – Promotes granulation and epithelial tissue growth – Improves blood circulation |
92% of ulcers healed after 8 weeks of treatment (vs. 32% in control group) |
| Pressure Ulcers | Occurs in areas under prolonged pressure (buttocks, heels) in bedridden patients | – Removes biofilm – Stimulates tissue regeneration and epithelialization – Reduces pain and inflammation |
55% reduction in ulcer size after 5–7 treatments |
| Venous Leg Ulcers | Chronic lower leg ulcers due to poor venous circulation | – Improves microcirculation via NO increase – Stimulates collagen and re-epithelialization – Reduces local swelling and inflammation |
Complete wound closure in 7/10 cases |
| Arterial Ulcers | Ulcers caused by arterial insufficiency, often on foot or lower leg | – Supports increased blood flow – Modulates immunity and reduces inflammation – Promotes new tissue formation in poorly perfused areas |
Caution required in areas with poor circulation; adjust exposure time accordingly |
| Surgical Wounds & Skin Grafts | Includes surgical incisions, donor/recipient graft sites | – Accelerates wound healing – Minimizes postoperative infection – Improves scar aesthetics |
Faster re-epithelialization with less scarring than control group |
| Burns | Thermal tissue damage (2nd–3rd degree burns), prone to infection | – Kills bacteria – Strongly reduces inflammation – Stimulates growth of new skin cells |
Accelerates healing and skin regeneration for both superficial and deep burns |
3. Procedure for Using Mirari Cold Plasma
Mirari Cold Plasma is designed for human use without any artificial or third-party products. Using other products simultaneously with Mirari Cold Plasma may cause unpredictable effects, harm, or injury. Please consult a healthcare professional before combining any products with Mirari Cold Plasma.
3.1. Patient and Treatment Area Preparation
Check Indications and Contraindications
Assess patient condition (not for patients with pacemakers, pregnant women, severe coagulation disorders, etc.).
Briefly discuss therapy goals with the patient.
Treatment Area Hygiene
Clean with water and mild soap, then pat dry.
Do not use alcohol or strong detergents directly before therapy. Cover open wounds with sterile gauze if necessary.
Initial Assessment
Record pain level (VAS), area or tissue characteristics, and check functional mobility if needed.
3.2. Device Preparation and Check
Clean Plasma Head and Accessories
Use a safe disinfectant solution and dry.
Install thermal insulation bag properly if indicated.
Check Power Supply and Connections
Ensure device has stable power, indicator lights or screen function correctly.
Set Treatment Mode
Select the correct mode (1: infection; 3: antiviral; 7: immunity…), place the Mirari plasma pad according to indication for each case.
Set therapy duration per area based on severity: Mild (15 min/session), Moderate–Severe (30 min/session), repeat according to protocol.
3.3. Therapy Administration
Position the Plasma Head Correctly
Place 0.5–1 cm above open wounds, or lightly touch soft tissue.
Keep the plasma head stable for the designated time per area. Treat multiple areas sequentially and record each location.
Monitor During Plasma Exposure
Observe skin response (warmth, redness, abnormal pain/tingling).
Stop treatment if discomfort occurs and recheck the area.
Do not continuously move the plasma head across multiple areas during the same session.
Maintain Optimal Distance and Time
Do not exceed recommended exposure time per treatment area.
Rest 5 minutes between different areas or if patient shows fatigue.
3.4. Post-Treatment
End Therapy Session
Turn off the device, allow natural cooling of skin (do not use ice packs directly).
Apply moisturizers if needed.
Record Post-Treatment Assessment
Reassess pain, patient sensation, tissue condition, swelling or exudate.
Document results for clinical progress tracking.
3.5. Device Maintenance & Cleaning
Clean Sensor Panels
Follow instructions on miraridoctor.vn.
Single-use thermal bags: replace after each session; ePTFE: dry and store in a clean, dry place.
Device Storage
Keep device away from moisture, avoid water/dust, store in box when not in use.
3.6. Safety Notes
Do not change therapy modes beyond physician protocol.
Do not combine cold plasma with untested medical cosmetics or topical corticosteroids unless prescribed.
4. Treatment Protocols for Musculoskeletal Conditions Using Mirari Cold Plasma
4.1. Key Biological Mechanisms of Mirari Cold Plasma in Wound Healing:
| Mechanism | Effect |
| Broad-Spectrum Antibacterial | Oxidizes bacterial membranes and DNA, eliminates biofilm. |
| Anti-Inflammatory | Reduces inflammatory cytokines (TNF-α), increases IL-10. |
| Growth Signaling | Stimulates VEGF, EGF → regenerates blood vessels and epithelium. |
| Tissue Cell Stimulation | Enhances fibroblast and keratinocyte activity. |
| Local NO Increase | Improves perfusion, collagen synthesis, antibacterial and anti-inflammatory effects. |
4.2. Mirari Cold Plasma Mode Configuration
| Protocol | Mode | |
| 1 | Infection | Antibacterial / Anti-inflammatory |
| 2 | Wound Healing | Promote Healing |
| 3 | Antiviral Therapy | Antiviral |
| 4 | Diabetes Therapy | Diabetes |
| 5 | Prostatitis Therapy | Prostate |
| 6 | Liver / Kidney Therapy | Liver / Kidney |
| 7 | Immunotherapy | Immune Support |
| 8 | Insomnia | Sleep Support |
| 9 | Arthritis | Arthritis |
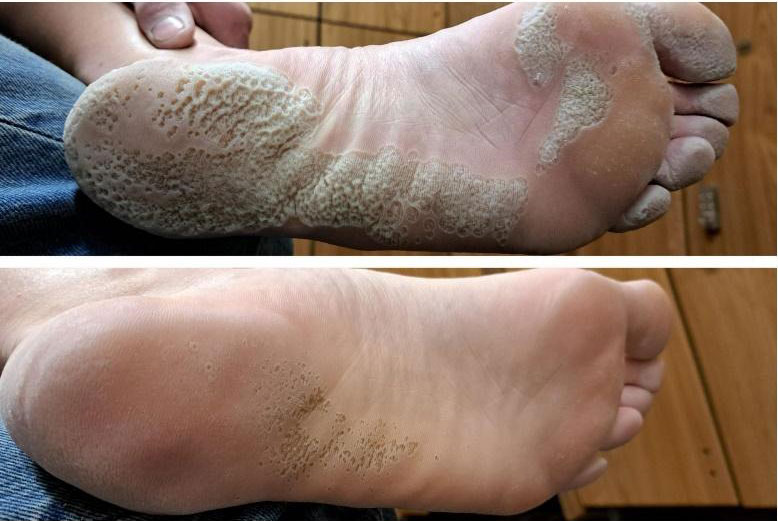
| 10 | Dermatitis/Fungus | Dermatitis / Fungal |

4.3. Mirari Cold Plasma Exposure Sites
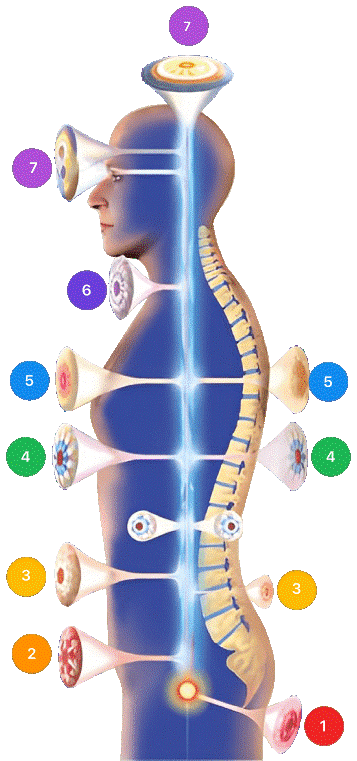
- Localized (0)
- Sacral (1)
- Prostate & Uterus (2)
- Kidneys, Liver & Spleen (3)
- Heart, Gallbladder & Pancreas (4)
- Lungs (5)
- Throat, Lymphatic & Thyroid (6)
- Nervous System & ENT (7)
4.4. Reference Treatment Protocol Using Mirari Cold Plasma Device
a) Supporting Burn Treatment
| Mild | Moderate | Severe |
| Mode: 1 (Infection) Position: 0 (Localized) Morning: 15 min, Evening: 15 min |
Mode: 1 (Infection) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min |
Mode: 1 (Infection) Position: 0 (Localized) Morning: 45 min, Noon: 45 min, Evening: 45 min |
| Mode: 2 (Wound Healing) Position: 0 (Localized) Morning: 15 min, Evening: 15 min |
Mode: 2 (Wound Healing) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min |
Mode: 2 (Wound Healing) Position: 0 (Localized) Morning: 45 min, Noon: 45 min, Evening: 45 min |
| Mode: 10 (Dermatitis/Fungal) Position: 0 (Localized) Morning: 15 min, Evening: 15 min |
Mode: 10 (Dermatitis/Fungal) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min |
Mode: 10 (Dermatitis/Fungal) Position: 0 (Localized) Morning: 45 min, Noon: 45 min, Evening: 45 min |
b) Supporting Wound Healing
| Mild | Moderate | Severe |
| Mode: 1 (Infection) Position: 0 (Localized) Morning: 15 min, Evening: 15 min |
Mode: 1 (Infection) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min |
Mode: 1 (Infection) Position: 0 (Localized) Morning: 45 min, Noon: 45 min, Evening: 45 min |
| Mode: 2 (Wound Healing) Position: 0 (Localized) Morning: 15 min, Evening: 15 min |
Mode: 2 (Wound Healing) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min |
Mode: 2 (Wound Healing) Position: 0 (Localized) Morning: 45 min, Noon: 45 min, Evening: 45 min |
5. Possible Side Effects
| Symptom | Severity | Management |
| Mild redness around wound | Mild – common | Resolves within hours, no intervention needed |
| Mild itching or tingling | Transient | Normal response; reassure patient |
| Peeling or dry skin at wound edges | Moderate | Apply moisturizer and monitor closely |
| Mild pain at treatment area | Rare | Reduce exposure time or increase distance of plasma head |
| Increased exudate in initial hours | Possible | Monitor and change sterile dressing frequently |
6. Important Notes on Using Mirari Cold Plasma
The Mirari Cold Plasma system is a Class 2 medical device, designed for use by trained healthcare professionals. It is registered in the USA, Thailand, and Vietnam.
The content provided with the device is for knowledge and educational purposes only and does not replace professional medical advice or care.
Device efficacy may vary by individual. No specific medical outcome is guaranteed.
Before using the device or relying on this information for treatment decisions, consult your physician or healthcare professional for the most appropriate protocol.
This guide is for informational purposes only and does not replace direct consultation with healthcare personnel. Please contact a qualified medical professional for diagnosis