Featured News, News
Announcement of GVM2-3.053 Version: Mirari® Cold Plasma Projection Device
To: Valued Customers, Partners, and All Relevant Individuals and Organizations
Mirari Import-Export Joint Stock Company is pleased to announce:
In order to fully comply with Clause a, Point 2, Article 52 of Decree 98/2021/ND-CP on medical equipment management and to ensure user safety, we officially recommend our partners and customers to only use version GVM2-3.053:
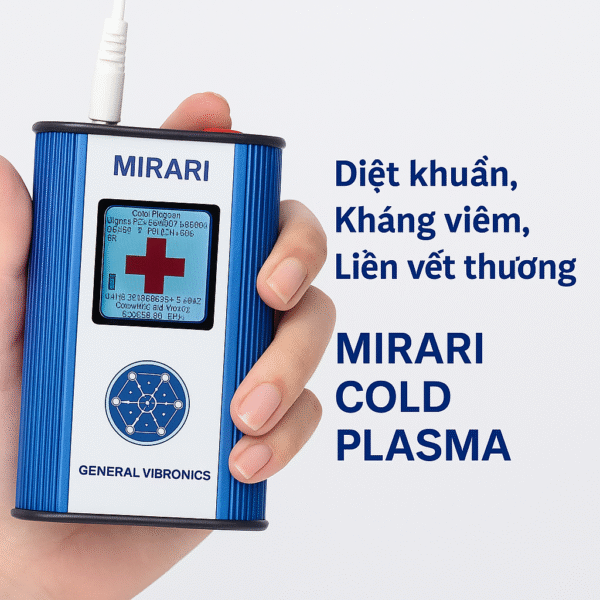
✅ FDA-Safe Version: Mirari® Cold Plasma Projection Device GVM2-3.053
- Manufactured by: General Vibronics Group – USA
- Approved by: U.S. FDA in November 2024
- Meets all international standards for safety and medical effectiveness in wound treatment, pain relief, dermatology, and musculoskeletal care.

Image of the Mirari Cold Plasma Projection Device version GVM2-3.053
⚠️ Warning: Older Devices May Not Be Safe
Mirari Cold Plasma Projection Devices prior to GVM2-3.053:
- Do not meet the quality standards under current FDA regulations.
- Are no longer guaranteed by Mirari Import-Export JSC for quality, effectiveness, or user rights after the upgrade program ends (March 2025).
- Touch arrays of Mirari Cold Plasma devices manufactured before version GVM2-3.053 are incompatible with GVM2-3.053 devices and vice versa.

Images of Mirari Cold Plasma devices from generations prior to GVM2-3.053
🔄 About the Upgrade Program
- From January 2025 to March 2025, Mirari Import-Export JSC implemented an upgrade program for customers using older versions.
- After this period, all non-upgraded devices no longer meet international safety and medical effectiveness standards.
CONTACT INFORMATION
For official product information, please contact:
Mirari JSC – Exclusive Distributor of MIRARI® in Vietnam
Address: 139 Hong Ha, Duc Nhuan Ward, Ho Chi Minh City
Website: https://miraridoctor.vn
Email: contact@miraridoctor.vn
Hotline: 0888 67 06 06
🎯 We sincerely thank our customers and partners for their continued cooperation and support!