News
Cold Plasma Mirari – A Breakthrough Solution for Wound Healing and Infection Control
In the context of hard-to-heal wounds such as diabetic foot ulcers, pressure sores, burns, post-surgical wounds, or chronic wounds becoming an increasing concern for the healthcare community, Cold Plasma Mirari emerges as an advanced, modern technology that brings great hope to both patients and healthcare professionals.
Why does Cold Plasma Mirari stand out?
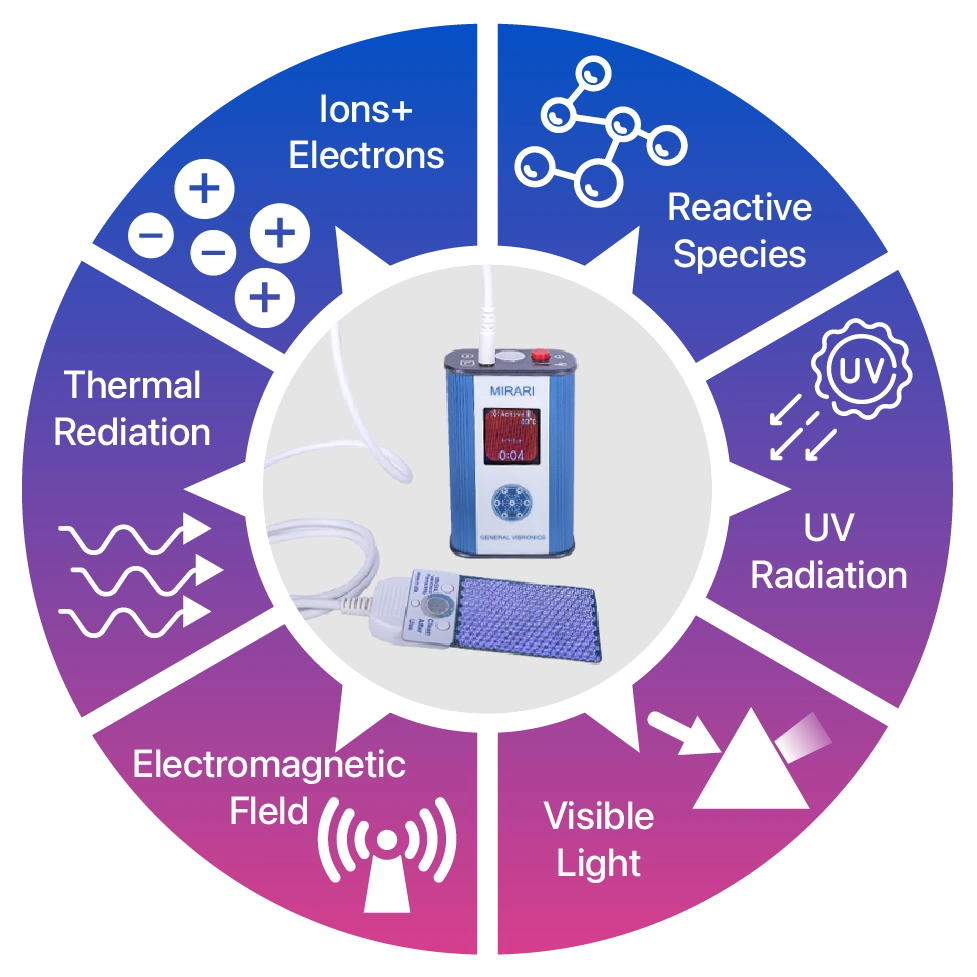
Cold Plasma Mirari belongs to the new generation applying Cold Atmospheric Plasma (CAP) technology, creating an environment rich in electrons, ions, and reactive species such as ROS (reactive oxygen species), RNS (reactive nitrogen species), along with electromagnetic waves and low-energy UV. It is completely safe for living tissue while extremely effective in destroying bacteria, viruses, and fungi. As a result, this is a non-invasive, low-risk medical device, approved in strict markets such as the USA (FDA Class II), Thailand, and Vietnam (Class B), suitable for wound care procedures in hospitals, healthcare facilities, and even at home.

Outstanding antimicrobial and anti-inflammatory effects
With the presence of powerful reactive components, Cold Plasma Mirari can inactivate a wide range of bacteria—including antibiotic-resistant strains—fungi, and viruses. This antimicrobial effect does not rely on antibiotics, thus avoiding drug resistance or long-term harmful side effects like many traditional methods. Moreover, Cold Plasma actively modulates inflammatory responses at the wound site, reducing swelling, redness, and pain, while creating optimal conditions for the body’s natural healing process.
Accelerating wound healing
Cold Plasma Mirari not only helps effectively control infection risks but also activates local biological processes such as increased growth factor production, stimulation of new blood vessel formation, and shortened granulation tissue regeneration time, thereby promoting epithelialization—a core factor in achieving stable wound closure. Clinical studies have shown that Mirari use accelerates wound closure, significantly reduces pain, and improves the final cosmetic outcome compared to traditional care.
Outstanding applications for hard-to-heal wounds
Cold Plasma Mirari demonstrates remarkable effectiveness in many challenging clinical scenarios, particularly complex, chronic wounds that are prone to complications.
- Diabetic foot ulcers: Cold Plasma Mirari helps reduce infection, stimulates healing of “stubborn” ulcers, and lowers the risk of amputation in diabetic patients.
- Pressure sores: Particularly suitable for the elderly and bedridden patients, Cold Plasma reduces the risk of secondary infections and prevents ulcer progression.
- Burns and post-surgical wounds: Helps reduce pain, prevents poor scarring and contractures, and shortens hospital stays.
- Chronic wounds and long-standing non-healing ulcers: Thanks to its antibacterial, anti-inflammatory, and multi-directional tissue regeneration effects, Mirari opens the door to healing for many patients who were previously “helpless” with conventional treatment.
Safe – Flexible – Easy to use
Cold Plasma Mirari provides an advanced treatment experience with outstanding advantages in convenience, safety, and user-friendliness, including:
- Compact handheld design, user-friendly interface, and easy operation even for first-time users of the technology.
- Does not damage healthy tissue, painless, and virtually no downtime after procedures.
- Clinically tested and confirmed to meet international safety standards with minimal risk of side effects.
Conclusion
Cold Plasma Mirari is a breakthrough in wound care, ideal for hard-to-heal ulcers, burns, post-surgical wounds, chronic wounds, diabetic ulcers, and pressure sores. With superior antimicrobial capability, proactive anti-inflammation, tissue regeneration stimulation, and high safety, this technology is increasingly applied in practice and delivering positive clinical outcomes.
References
- Fridman, G., Peddinghaus, M., Ayan, H., Fridman, A., Balasubramanian, M., Gutsol, A., Brooks, A., & Friedman, G. (2006). Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry and Plasma Processing, 26(4), 425-442. https://doi.org/10.1007/s11090-006-9024-4
- Heinlin, J., Zimmermann, J. L., Zeman, F., Bunk, W., Isbary, G., Landthaler, M., Maisch, T., Monetti, R., Morfill, G., Shimizu, T., Steffes, B., Karrer, S. (2013). Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair and Regeneration, 21(6), 800-807. https://doi.org/10.1111/wrr.1207
- Julia Heinlin; Gregor Morfill; Michael Landthaler; Wilhelm Stolz; Georg Isbary; Julia L. Zimmermann; Tetsuji Shimizu; Sigrid Karrer (2010). Plasma medicine: possible applications in dermatology. https://doi.org/10.1111/j.1610-0387.2010.07495.x
- J Heinlin; G Isbary; W Stolz; G Morfill; M Landthaler; T Shimizu; B Steffes; T Nosenko; JL Zimmermann; S Karrer (2011). Plasma applications in medicine with a special focus on dermatology. https://doi.org/10.1111/j.1468-3083.2010.03702.x
- Maisch, T.; Shimizu, T.; Isbary, G.; Heinlin, J.; Karrer, S.; Klampfl, T. G.; Li, Y.-F.; Morfill, G.; Zimmermann, J. L. (2012). Contact-Free Inactivation of Candida albicans Biofilms by Cold Atmospheric Air Plasma. https://doi.org/10.1128/AEM.07235-11
- Laroussi M, Leipold F Evaluation of the Roles of Reactive Species, Heat, and UV Radiation in the Inactivation of Bacterial Cells by Air Plasmas at Atmospheric Pressure. Int J Mass Spectrom (2004). https://doi.org/10.1016/j.ijms.2003.11.016
- Brandenburg R, Lange H, Von Woedtke T, Stieber M, Kindel E, Ehlbeck J, et al. Antimicrobial Effects of UV and VUV Radiation of Nonthermal Plasma Jets. IEEE Trans Plasma Sci (2009). https://doi.org/10.1109/tps.2009.2019657
- Schneider S, Lackmann J-W, Ellerweg D, Denis B, Narberhaus F, Bandow JE, et al. The Role of VUV Radiation in the Inactivation of Bacteria with an Atmospheric Pressure Plasma Jet. Plasma Process. Polym (2012). https://doi.org/10.1002/ppap.201100102
- Lange H, Foest R, Schafer J, Weltmann K-D Vacuum UV Radiation of a Plasma Jet Operated with Rare Gases at Atmospheric Pressure. IEEE Trans Plasma Sci (2009). https://doi.org/10.1109/tps.2009.2019982
- Schröder, D ; Bahre, H ; Knake, N ; Winter, J ; de los Arcos, T ; Schulz-von der Gathen, V (2012). Influence of target surfaces on the atomic oxygen distribution in the effluent of a micro-scaled atmospheric pressure plasma jet. https://doi.org/10.1088/0963-0252/21/2/024007
- Rastogi RP, RichaKumar A, Kumar A, Tyagi MB, Sinha RP. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J Nucleic Acids (2010). https://doi.org/10.4061/2010/592980
- Mikkelsen RB, Wardman P Biological Chemistry of Reactive Oxygen and Nitrogen and Radiation Induced Signal Transduction Mechanisms. Oncogene (2003). https://doi.org/10.1038/sj.onc.1206663
- Liedtke, Kim Rouven ; Bekeschus, Sander ; Kaeding, André ; Hackbarth, Christine ; Kuehn, Jens-Peter ; Heidecke, Claus-Dieter ; von Bernstorff, Wolfram ; von Woedtke, Thomas ; Partecke, Lars Ivo (2017). Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. https://doi.org/10.1038/s41598-017-08560-3
- Daeschlein, Georg ; Scholz, Sebastian ; Arnold, Andreas ; von Podewils, Sebastian ; Haase, Hermann ; Emmert, Steffen ; von Woedtke, Thomas ; Weltmann, Klaus-Dieter ; Jünger, Michael (2012). In Vitro Susceptibility of Important Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). https://doi.org/10.1002/ppap.201100160
- Weltmann, K-D ; Brandenburg, R ; von Woedtke, T ; Ehlbeck, J ; Foest R ; Stieber, M ; Kindel, E (2008). Antimicrobial Efficacy and Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs). https://doi.org/10.1002/ctpp.200710011
- YijiaoWu, Shiyu Yu, Xiyin Zhang, Xianzhong Wang and Jiaojiao Zhang. The Regulatory Mechanism of Cold Plasma in Relation to Cell Activity and Its Application in Biomedical and Animal Husbandry Practices (2023). https://doi.org/10.3390/ijms24087160
- ATT 3_Section 13_Biocompatibility_Compiled
- ATT 5_Section 15_EMC_and_Safety_Compiled-1
- ATT 6_Section 16_Performance Testing-Bench_Compiled-Final-1
- U.S. Food and Drug Administration. (2020, June 19). Use of International Standard ISO 10993-1, “Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process”. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and