Applications of Mirari Cold Plasma
Dermatology Support Application
MIRARI COLD PLASMA APPLICATION IN SUPPORTING DERMATOLOGICAL TREATMENT
1. General Information
Mirari Cold Plasma is a cold plasma generator. Cold plasma is a low-energy state of matter containing charged particles, activated molecules, and ultraviolet light at a safe level. When applied to skin/tissue, cold plasma can kill bacteria, reduce inflammation, promote tissue regeneration, and relieve swelling and pain without thermal damage, making it effective for musculoskeletal diseases, wounds, dermatology, and functional recovery.
Mirari Cold Plasma is certified as a Class B medical device in Vietnam and is FDA-approved for safety and quality standards.
Mirari Cold Plasma is developed by General Vibronics in the USA and exclusively distributed by Mirari Import-Export Joint Stock Company in Vietnam.
2. Applications of Mirari Cold Plasma in Dermatological Treatment
The Mirari Cold Plasma device is designed to support the treatment of dermatological conditions, including:
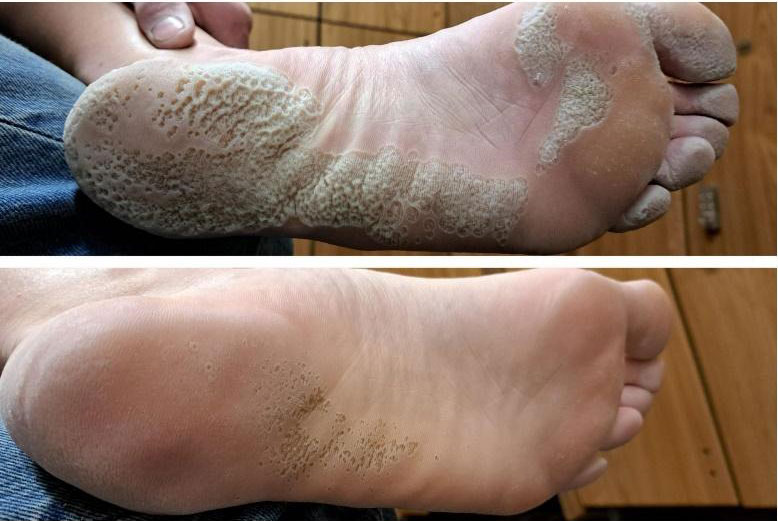
| Dermatological Condition | Clinical Manifestation | Mechanism of Mirari Cold Plasma | Clinical Evidence / Research |
| Acne | Follicular inflammation, whiteheads, blackheads, papules, pustules | – Antibacterial (Propionibacterium acnes) – Reduce inflammatory cytokines – Modulate skin microbiota |
RCT studies show significant reduction in acne count and improved skin appearance after a few weeks of treatment |
| Allergic Dermatitis (Eczema) | Dry, red, itchy skin, often in children and allergic individuals | – Soothes inflammation – Increases IL-10 expression – Restores skin barrier – Enhances skin hydration |
CAP improves skin barrier without reducing moisture |
| Psoriasis | Red plaques with silver scales, itching, inflammation, recurrent | – Immune modulation – Reduce IL-6, TNF-α – Inhibit keratinocyte proliferation – Enhance cell differentiation |
Grafted skin studies show reduced inflammatory cytokines and tissue recovery |
| Rosacea | Facial redness, pustules, telangiectasia | – Reduce inflammation – Improve skin circulation – Antimicrobial (Demodex, associated bacteria) |
Reduces redness, vessel dilation, and improves aesthetics after several sessions |
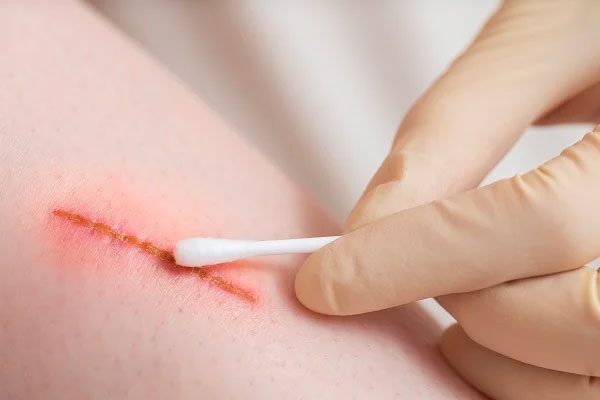
| Skin Infections | Bacterial (Staphylococcus, Streptococcus), viral (Herpes), fungal (Candida, Trichophyton) | – Broad-spectrum antimicrobial (bacteria, fungi, viruses) – Biofilm disruption – No antibiotic resistance |
Effective against MRSA, Candida, HSV in vitro and animal models |
| Skin Aging (Rejuvenation) | Wrinkles, sagging, pigmentation | – Stimulates fibroblasts – Increases collagen synthesis – Improves elasticity and epidermal thickness |
Increases collagen and improves skin structure after 4–6 sessions |
| Hyperpigmentation / Pigment Disorders | Dark, sun-damaged, uneven skin tone | – Affects tyrosinase and melanin – Regulates pigmentation biologically |
Plasma-derived NO regulates melanin synthesis in melanocytes |
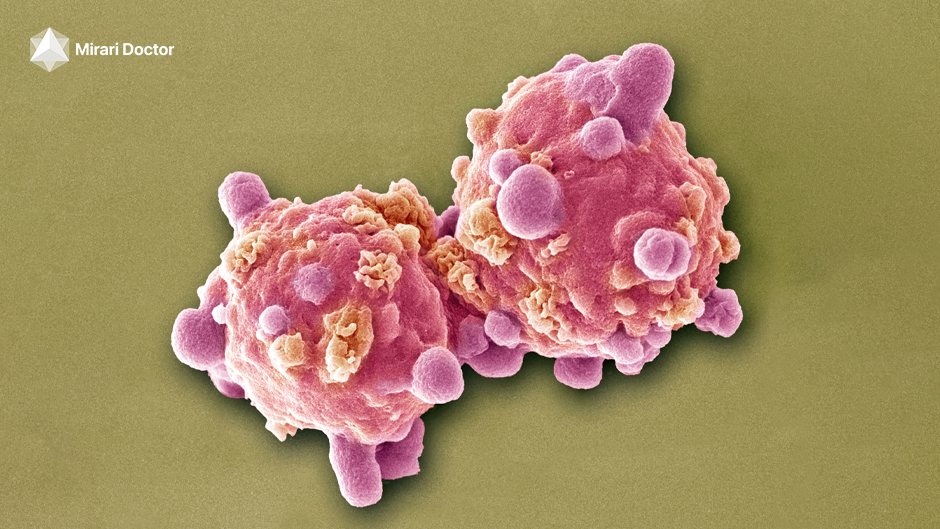
| Skin Cancer (Research Support) | Basal cell carcinoma, squamous cell carcinoma, melanoma | – Selective cancer cell apoptosis via ROS/NO – No damage to healthy tissue – Potential alternative to laser or radiotherapy |
Preclinical studies demonstrate selective efficacy against melanoma |
3. Mirari Cold Plasma Treatment Procedure
The Mirari Cold Plasma device is designed for human use without any artificial or third-party products. Combining with other products may cause unforeseen harmful effects. Please consult a healthcare professional before combining treatments.
3.1. Patient and Treatment Area Preparation
Check indications and contraindications: Assess patient status (not for pacemaker users, pregnant women, severe coagulation disorders, etc.). Discuss therapy goals briefly with the patient.
Clean treatment area: Wash gently with water and mild soap, pat dry. Avoid alcohol or harsh chemicals. Cover open wounds with sterile gauze.
Initial assessment: Record pain level (VAS), lesion area or characteristics, and assess function if needed.
3.2. Device Preparation and Check
Clean plasma head and accessories: Use safe disinfectant and dry. Attach thermal bag properly if indicated.
Check power and connection: Ensure stable power supply, indicator lights, and screen function. Set treatment mode according to condition: 1 (infection), 3 (antiviral), 7 (immune), etc. Set treatment duration per area: Mild (15 min), Moderate–Severe (30 min), repeat as prescribed.
3.3. Treatment Execution
Position plasma head: 0.5–1 cm from open wound, or light contact if soft tissue. Keep stable for prescribed duration. Treat multiple areas sequentially and record locations.
Monitor during plasma exposure: Observe skin reaction (warmth, redness, unusual pain/prickling). Stop if discomfort occurs and check area. Avoid moving head continuously across multiple areas simultaneously.
Maintain optimal distance and time: Do not exceed recommended duration per area. Rest 5 min between areas or if patient shows fatigue.
3.4. Post-treatment
End session: Turn off device, allow natural cooling (no direct ice). Apply moisturizer if needed. Record post-treatment pain, patient feedback, tissue status, swelling or exudate. Maintain treatment log for clinical evaluation.
3.5. Device Maintenance
Clean sensor: Follow manufacturer instructions at miraridoctor.vn. For disposable thermal bags, replace each session; for ePTFE, dry and store in a clean, dry place.
Storage: Avoid moisture, dust, and water. Pack in box when not in use.
3.6. Safety Notes
Do not alter therapy settings outside prescribed protocol. Avoid combining with unverified medical cosmetics or topical corticosteroids unless prescribed.
4. Mirari Cold Plasma Supportive Treatment Protocol for Dermatology
4.1. Key Biological Mechanisms:
- Antibacterial & antiviral: Kills drug-resistant bacteria, HSV, HPV.
- Anti-inflammatory: Reduces cytokines (IL-1, TNF-α), increases IL-10.
- Promotes healing: Stimulates keratinocytes & fibroblasts, increases EGF, VEGF.
- Localized nitric oxide: Improves circulation, angiogenesis, pigment regulation.
No thermal damage: Safe for thin, sensitive skin (face, neck).
4.2. Device Modes Configuration:
| Protocol | Mode | |
| 1 | Infection | Antibacterial / Anti-inflammatory |
| 2 | Wound Healing | Healing |
| 3 | Antiviral Therapy | Virus therapy |
| 4 | Diabetes Therapy | Diabetes |
| 5 | Prostatitis Therapy | Prostate |
| 6 | Liver / Kidney Therapy | Liver/Kidney |
| 7 | Immunotherapy | Immune support |
| 8 | Insomnia | Sleep disorder |
| 9 | Arthritis | Arthritis |
| 10 | Dermatitis/Fungus | Dermatitis/Fungus |
4.3. Plasma Application Sites:
- Localized (0)
- Sacrum (1)
- Prostate & uterus (2)
- Kidney, Liver & Spleen (3)
- Heart, Gallbladder & Pancreas (4)
- Lungs (5)
- Throat, Lymph & Thyroid (6)
- Nervous system & ENT (7)
4.4. Sample Treatment Protocols
For Acne:
| Mild | Moderate | Severe |
| Mode: 1 (Infection) Position: 0 (Localized) Morning: 15 min, Evening: 15 min |
Mode: 1 (Infection) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min |
Mode: 1 (Infection) Position: 0 (Localized) Morning: 45 min, Noon: 45 min, Evening: 45 min |
5. Possible Side Effects (mostly mild and transient)
| Side Effect | Severity | Management |
| Mild redness | Transient | Resolves in a few hours |
| Prickling sensation | Mild | Stop treatment if prolonged |
| Dry or peeling skin | Mild – Moderate | Regular moisturizing |
| Temporary pigmentation changes | Rare | Self-resolving, monitor pigment |
6. Important Notes on Using Mirari Cold Plasma
Mirari Cold Plasma is a Class 2 medical device designed for trained healthcare professionals. Registered in the USA, Thailand, and Vietnam. The information provided is for educational purposes and does not replace professional medical advice or treatment.
Results may vary individually. Consult a physician or healthcare professional for proper guidance. This guide is for informational purposes and should not replace direct medical consultation.