Applications of Mirari Cold Plasma
Pain Relief Support Application
MIRARI COLD PLASMA APPLICATION FOR PAIN RELIEF SUPPORT
1. General Information
Mirari Cold Plasma is a cold plasma generator. Cold plasma is a low-energy state of matter containing charged particles, activated molecules, and safe-level UV rays. When applied to skin/tissue, cold plasma can disinfect, reduce inflammation, promote tissue regeneration, and relieve swelling and pain without causing thermal damage, making it effective for musculoskeletal disorders, wounds, dermatology, and rehabilitation.
Mirari Cold Plasma is certified as a Class B medical device in Vietnam and has FDA approval from the U.S. Food and Drug Administration for safety and quality standards.
Mirari Cold Plasma is developed by General Vibronics in the U.S. and exclusively distributed in Vietnam by Mirari Import-Export Joint Stock Company.
2. Applications of Mirari Cold Plasma in Pain Relief Support
The Mirari Cold Plasma device is designed to support pain relief, including:
| Condition / Pain Type | Characteristics / Clinical Description | Mechanism of Mirari Cold Plasma | Representative Clinical Evidence |
| Musculoskeletal Pain | Osteoarthritis, back pain, joint pain, tennis elbow, myofascial pain syndrome | – Reduces inflammation (IL-6, TNF-α) – Promotes cartilage & tendon regeneration – Stimulates fibroblasts – Increases blood circulation |
RCT studies on knee and elbow arthritis: significant pain reduction and improved function |
| Neuropathic Pain | Postherpetic neuralgia, diabetic neuropathy | – Modulates nociceptor activity – Reduces central and peripheral sensitivity – Enhances endogenous opioid release – Promotes nerve tissue regeneration |
Case series and RCT: 50–100% pain reduction, lasting up to 3 months |
| Postoperative Pain | After knee replacement, tooth extraction, soft tissue surgery | – Reduces inflammation – Limits edema – Prevents infection – Accelerates wound healing |
RCT: reduced opioid use, faster recovery, higher satisfaction |
| Non-specific Chronic Pain | Chronic lower back pain, fibromyalgia | – Suppresses neuroinflammation – Stabilizes HPA axis and neural signaling – Regulates endogenous nitric oxide |
Pilot studies: reduced pain intensity and disability scores after 4–8 weeks |
| Inflammatory / Immune Pain | Soft tissue inflammation, arthritic pain, tendinitis | – Reduces pro-inflammatory cytokines – Increases IL-10, TGF-β – Limits oxidative stress |
In vivo and in vitro evidence in mice & human tissues |
3. Mirari Cold Plasma Usage Procedure
Mirari Cold Plasma is designed for human use without any artificial or third-party products. Using other products alongside Mirari Cold Plasma may cause unpredictable effects, harm, or injury. Consult a healthcare professional before combining any other product with Mirari Cold Plasma.
3.1. Patient and Treatment Area Preparation
Check indications and contraindications: Assess patient status (not for pacemaker users, pregnant women, severe bleeding disorders, etc.). Briefly discuss therapy goals with the patient.
Clean the treatment area: Wash with mild soap and water, pat dry. Do not apply alcohol or strong cleansers directly before therapy. Cover open wounds with sterile gauze.
Initial Assessment: Record pain level (VAS), area or characteristics of tissue damage, and check mobility if needed.
3.2. Device Preparation and Check
Clean plasma tip and accessories: Use safe disinfectant solution and dry. Fit thermal protection bags if indicated.
Check power supply and connections: Ensure stable power, indicators, and screen function properly.
Set treatment mode: Choose appropriate mode number (1: infection, 3: antiviral, 7: immune…), place plasma pad according to instructions. Set treatment time per area based on symptom severity: Mild (15 min/session), Moderate–Severe (30 min/session), repeat per protocol.
3.3. Conduct Therapy
Place tip in correct position: 0.5–1 cm above open wounds or gently touch soft tissue. Maintain position for prescribed time per area. If treating multiple areas, proceed sequentially and record each location.
Monitor during plasma exposure: Observe skin reaction (warmth, redness, unusual pain/tingling). Stop therapy if discomfort occurs and recheck area. Do not sweep tip continuously across multiple areas during one session.
Maintain optimal distance and duration: Do not exceed recommended time per area. Rest 5 min between areas or if patient shows fatigue.
3.4. Post-Treatment
End session: Turn off device, allow natural cooling (do not use ice packs directly). Apply moisturizing products if needed.
Post-treatment assessment: Reassess pain, patient feedback, tissue condition, swelling or exudate. Record results for next session evaluation.
3.5. Device Maintenance and Cleaning
Clean plasma pad: Follow company guidelines at miraridoctor.vn. Single-use thermal bags: replace after each session; for ePTFE, dry and store in a clean, dry place.
Device storage: Avoid moisture, water/dust contact; store in case when not in use.
3.6. Important Safety Notes
Do not alter therapy mode outside physician protocol. Avoid combining cold plasma with unverified medical cosmetics or topical corticosteroids unless prescribed.
4. Pain Relief Support Protocols
4.1. Key Biological Mechanisms of Mirari Cold Plasma in Pain Relief:
- Anti-inflammatory: Inhibits TNF-α, IL-6; increases IL-10, TGF-β
- NO Production: Vasodilation, improved blood flow, pain modulation
- Direct Neural Effects: Reduces nociceptor activation, improves nerve conduction
- Oxidative Stress Protection: Protects damaged tissue, enhances recovery
- Tissue Regeneration: Increases collagen, fibroblasts, and angiogenesis
4.2. Mirari Cold Plasma Mode Configuration
| Protocol | Mode | |
| 1 | Infection | Disinfection / Anti-inflammatory |
| 2 | Wound Healing | Wound Repair |
| 3 | Antiviral Therapy | Virus Therapy |
| 4 | Diabetes Therapy | Diabetes Treatment |
| 5 | Prostatitis Therapy | Prostate Treatment |
| 6 | Liver / Kidney Therapy | Liver / Kidney Treatment |
| 7 | Immunotherapy | Immune Enhancement |
| 8 | Insomnia | Sleep Improvement |
| 9 | Arthritis | Arthritis |
| 10 | Dermatitis/Fungus | Dermatitis / Fungal Treatment |

4.3. Mirari Cold Plasma Application Sites
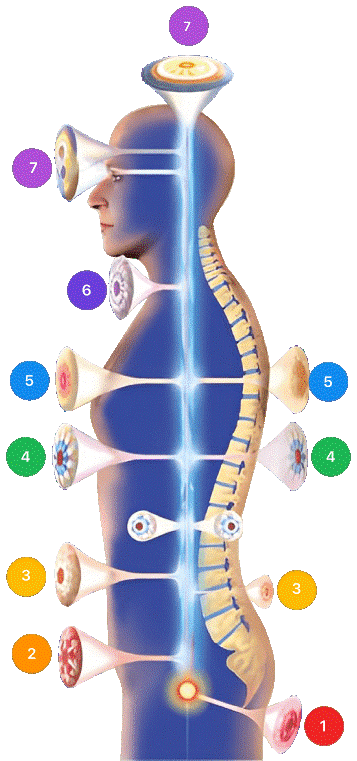
- Localized (0)
- Sacrum (1)
- Prostate & Uterus (2)
- Kidney, Liver & Spleen (3)
- Heart, Gallbladder & Pancreas (4)
- Lungs (5)
- Throat, Lymphatic System & Thyroid (6)
- Nervous System & ENT (7)
4.4. Reference Treatment Protocol Using Mirari Cold Plasma for Pain Relief
| Mild | Moderate | Severe |
| Mode: 1 (Infection) Position: 0 (Localized) Morning: 15 min, Evening: 15 min | Mode: 1 (Infection) Position: 0 (Localized) Morning: 30 min, Noon: 30 min, Evening: 30 min | Mode: 1 (Infection) Position: 0 (Localized) Morning: 45 min,
Chia Sẻ:
|