Featured News, News
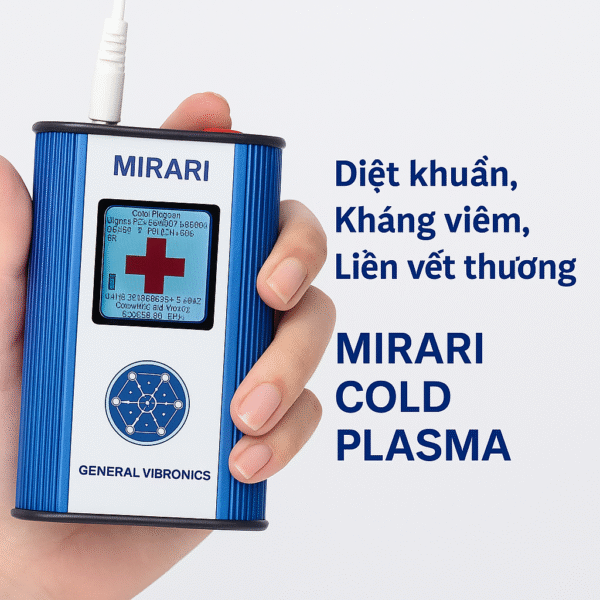
Exclusive Distributor of Mirari Cold Plasma in Vietnam
Mirari Cold Plasma Technology from the United States has been making breakthroughs in the medical and aesthetic fields, enabling thousands of patients and customers to access non-invasive, safe, and effective treatment methods. In Vietnam, Mirari Import-Export Joint Stock Company serves as the exclusive distributor of this device, ensuring the supply of genuine solutions with full legal compliance and comprehensive technical support.
- Transparent legal framework – Guaranteeing exclusive distribution rights in Vietnam
The official website miraridoctor.vn is the sole web address of Mirari Import-Export JSC in Vietnam, responsible for exclusively distributing the Mirari Cold Plasma device. The company has obtained all required legal permits under Vietnamese law and holds official distribution certification from General Vibronics (USA) – the technology owner and FDA-approved producer of the Mirari Cold Plasma GVM2-3.053 version.

- Key features of the Mirari Cold Plasma device manufactured by General Vibronics
- Natural gas source: The Mirari device uses ambient air, concentrating on generating Nitric Oxide (NO) as the main therapeutic agent, promoting vasodilation, anti-inflammation, and tissue recovery. This is a key differentiator compared to plasma devices using argon gas, which require bulky, pure gas cylinders and more complex operation.
- High mobility: Mirari is compact and operates without external compressed gas, making it easy to use in clinics, mobile healthcare facilities, and cost-effective.
- Safe for tissue and skin: Cold plasma temperature stays below 40°C, reducing the risk of burns or tissue damage compared to other plasma devices.
- Clinical effectiveness from hospital workshops
Workshops at Hospital 108 provided substantial scientific evidence supporting the superior effectiveness of Mirari Cold Plasma:
- Mirari Cold Plasma promotes wound healing and reduces inflammation through controlled Nitric Oxide (NO) release, which both increases blood flow to injured areas and regulates inflammatory response. This controlled NO release mechanism also underpins fast pain and swelling reduction, enhancing tissue repair efficiency.
- Reduces swelling and pain by over 50% in a single session, significantly improving patient experience.
- Positive evaluations regarding safety, convenience, and applicability across diverse patient groups, including dermatology, musculoskeletal, and post-aesthetic surgery recovery.
Hospitals such as Hoè Nhai Hospital, E Hospital, and many other medical facilities in Vietnam have implemented Mirari Cold Plasma technology, reporting positive treatment outcomes:
- The technology effectively eliminates bacteria and reduces inflammation, ensuring a clean treatment area and minimizing infection risk in wound care, dermatology, and chronic conditions.
- Mirari Cold Plasma accelerates wound healing, stimulates collagen production, regenerates cells, and repairs damaged tissue, significantly shortening recovery time for post-surgery or chronic skin patients.
- The device also noticeably reduces swelling and pain immediately after treatments, enhancing patient quality of life.
- Notably, the device operates safely below 40°C without causing burns or tissue damage, suitable for sensitive patients.
- Doctors and experts at these hospitals evaluate Mirari Cold Plasma technology as having broad potential applications across medical fields, from skincare to musculoskeletal therapy and post-aesthetic surgery recovery.
Clinical studies presented at workshops and published in international medical journals confirm its significant effectiveness, with swelling and pain reductions exceeding 50% after just one treatment session. These metrics are based on patient recovery tracking using Mirari Cold Plasma in officially collaborating hospitals.
- How to verify the origin of the Mirari Cold Plasma device
Customers and healthcare facilities wishing to verify authentic Mirari Cold Plasma products can:
- Visit the official website miraridoctor.vn for product information and email contact@miraridoctor.vn to validate serial numbers and authenticity certificates of Mirari Cold Plasma devices.
- Check the FDA certificate from General Vibronics via the official U.S. FDA website or request a copy from Mirari Import-Export JSC, the authorized representative in Vietnam.
- Verify labels, user manuals, and accompanying invoices or documents when purchasing the product and/or plasma module.
Contact Mirari Import-Export JSC via the official website: miraridoctor.vn to receive consultation and explore the most advanced and safe cold plasma technology available today!
CONTACT INFORMATION
For official product information, please contact
Mirari Import-Export JSC – Exclusive distributor of MIRARI® in Vietnam
Address: 139 Hong Ha, Duc Nhuan Ward, Ho Chi Minh City
Website: https://miraridoctor.vn
Email: contact@miraridoctor.vn
Hotline: 0888 67 06 06
🎯 We sincerely thank our customers and partners for their cooperation and support throughout the years!