Featured News, News
The Differences Between Generations and Technical Specifications of the MIRARI® Cold Plasma System
1. Introduction
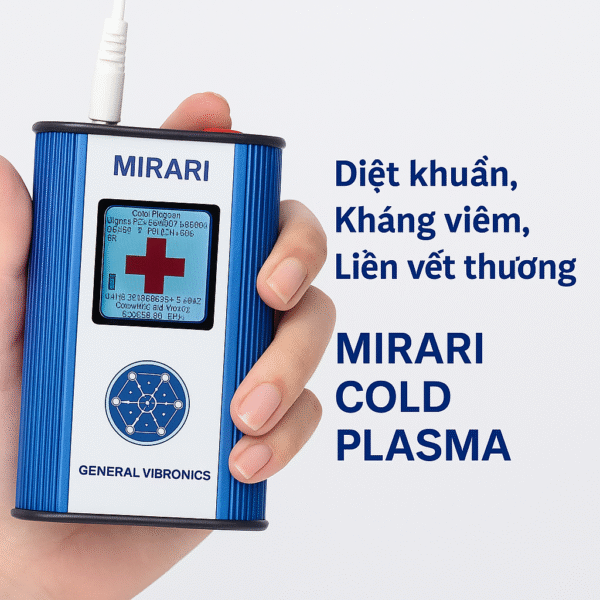
The MIRARI® Cold Plasma System represents a revolutionary breakthrough in medical technology. As the world’s first handheld atmospheric cold plasma (CAP) device, this advanced system has transformed the way medical professionals approach wound healing, pain management, and dermatological treatments.
Developed and patented by General Vibronics Inc. in the United States, the MIRARI system has evolved through multiple generations. Each version offers enhanced clinical performance, extended application time, along with greater convenience and safety for users.
But what truly differentiates each generation? And how do these technical specifications impact real-world clinical outcomes?
2. The Development Journey of MIRARI® Generations: A Transformative Path
2.1. Device development over application time
The MIRARI Cold Plasma System has undergone remarkable transformation across five distinct generations. Each version meets specific clinical needs while being built on a proven plasma therapy foundation.
| Generation | Application Time | Main Application | Key Improvements |
| GVM1 | 240 minutes | Clinical research (basic dermatology) | Foundational CAP technology |
| GVM1 | 360 minutes | Research-focused with improved durability | Enhanced battery and plasma array technology |
| GVM1 | 480 minutes | Outpatient clinics and therapy | Dual treatment mode |
| GVM1 | 600 minutes | Clinics & hospitals, modernized design | Ergonomic improvements |
| GVM2 / 3.053 | 1200 minutes | Comprehensive hospital-level, multidisciplinary use | FDA-approved & fully compliant |
2.2. Regulatory milestones that reshaped the landscape
In November 2024, the GVM2 / 3.053 version reached a historic milestone. This device received FDA approval—a testament to its safety, performance, and efficacy.
This certification validates the system’s effectiveness in wound healing and dermatological applications. More importantly, it opened the door for widespread clinical adoption across hospital networks and specialized medical centers.

3. Technical Specifications by Generation: Engineering Marvels
3.1. Core components powering every version
All MIRARI Cold Plasma Systems share fundamental components that ensure consistent treatment results:
- Plasma Driver: The heart of the system. This rechargeable control box contains an RF/DBD plasma generator to produce therapeutic plasma fields.
- Plasma Array: Electrode-based CAP emitter, sized 2.50″ x 1.72″. This replaceable component generates thousands of micro-plasma streams across the printed circuit board.
- Insulation Bag:
- Waterproof ePTFE bag for moisture protection
- Felt thermal bag for temperature control
- Power Supply: Modern USB-C charging with optimized battery capacity for each generation’s runtime requirements.
3.2. Operational characteristics: Precision engineering in action
| Specification | Value / Description | Clinical Impact |
| RF Frequency | 80 kHz (unipolar) | Optimal tissue penetration |
| Plasma Generation Method | Dielectric Barrier Discharge (DBD) | Safe, non-thermal plasma generation |
| Output Voltage (Peak-to-Peak) | Requirement >700V to generate CAP | Sufficient ionization for therapeutic efficacy |
| Temperature Limit | Maximum surface temperature: 43°C | Patient comfort and safety |
| UV Emission | >99% UV-A spectrum (310–470 nm) | Antimicrobial effect without harmful radiation |
| Ozone Emission | <40 ppb (well below UL 867 limit) | Compliant with environmental safety |
3.3. Safety features: Multi-level protection
The MIRARI system integrates multiple safety mechanisms:
- Automatic shutdown in case of short circuit, overheating, or arc discharge
- Capacitive & resistive thermal modulation based on tissue type
- Integrated sensors and temperature feedback control
These features ensure patient safety while maintaining treatment efficacy across all protocols.
4. Distinctive Features Across Versions: What Makes Each Generation Unique
| Feature | 240 minutes | 360 minutes | 480 minutes | 600 minutes | 1200 minutes |
| Main Application | Beauty salons | Beauty salons | Clinics | Clinics/Hospitals | Hospitals/Certified use |
| Hardware Upgrades | Basic CAP array | Improved battery | Enhanced design | Ergonomic improvements | FDA-approved specifications GVM2 Software 3.053 |
| Runtime | 240 minutes | 360 minutes | 480 minutes | 600 minutes | 1200 minutes |
| User Type | Physicians | Physicians | Physicians | Clinics | Hospitals |
| Regulatory Readiness | Pre-market | Pre-market | Clinical-level for FDA certification | Ready for FDA certification | FDA-approved |
| System Safety & Feedback | Basic | Intermediate | Advanced | Advanced | Fully compliant system |